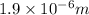Answer: The maximum wavelength of light that can be emitted is
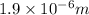
Step-by-step explanation:
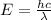
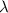 = Wavelength of radiation
= Wavelength of radiation
E= energy
For wavelength to be maximum, energy would be minimum, i.e the electron will jump from n=4 level to n =3
Using Rydberg's Equation:
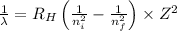
Where,
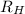 = Rydberg's Constant =
= Rydberg's Constant =
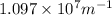
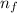 = Higher energy level = 3
= Higher energy level = 3
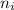 = Lower energy level = 4
= Lower energy level = 4
Z= atomic number = 1 (for hydrogen)
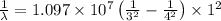
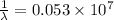
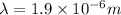
Thus the maximum wavelength of light that can be emitted is