Answer:
Maximum height the atmosphere pressure can support the
water=10.336 m
Step-by-step explanation:
We know that ,
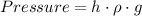
Case 1 - Mercury in the tube
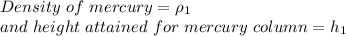
Case 2 - Water in the tube
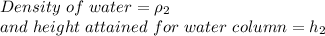
Since atmospheric pressure is same
.
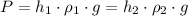
or,
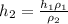
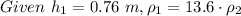
∴
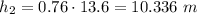
Hence height of the water column =10.336 m