The molarity of sucrose solution is 0.19 M.
The molarity of HCl is 12.8 M.
Step-by-step explanation:
a. Molarity can be found by finding its moles and volume of water in L and then dividing both(moles divided by volume in Litres).
Mass of sucrose = 318. 6 g
Molar mass of sucrose = 342.3 g/mol
Moles =
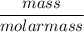
=
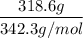
= 0.93 moles
Mass of water = 4905 g
Density of water = 1000 g/L
Volume =
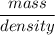
=
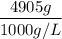
= 4.905 L
Now we can find the molarity =
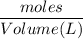
=
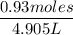
= 0.19 M
So the molarity of sucrose solution is 0.19 M.
b. The molarity of HCl can be found as follows.
It is given that 39% HCl that means it contains 39 g of acid in 100 g of water.
Density of the solution is 1.20 g/mL, from this mass can be found as,
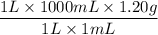
= 1200 g
Now we have to find out the amount of HCl in grams as,
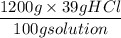
= 468 g HCl
Now we have to find the number of moles,
moles =
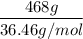
= 12.8 moles
Molarity of HCl =
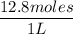
= 12.8 M
So the molarity of HCl is 12.8 M.