Answer:
0.17094 liters
Step-by-step explanation:
-For STP, Pressure=1 atm and Temperature=273K
-We apply the combined gas law which relates temperature, pressure and Volume:
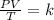
-We therefore equate the relationship at different temperatures as follows:
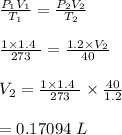
Hence, the new volume is 0.17094 liters