Answer : The required bond energy per mole for breaking all the bonds in methane is, 1656 kJ/mol
Explanation :
The given compound is,
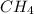 .
.
Methane compound breakdown into 4 C-H bonds.
Given:
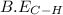 = 414 kJ/mol
= 414 kJ/mol
The expression will be:
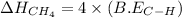
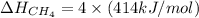
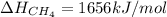
Therefore, the required bond energy per mole for breaking all the bonds in methane is, 1656 kJ/mol