The molarity of the HCl is 1 M when 12.0 of .500 M NaOH neutralized 6.0 ml of HCl solution.
Step-by-step explanation:
Data given:
molarity of the base NaOH, Mbase =0. 5 M
volume of the base NaOH, Vbase = 12 ml
volume of the acid, Vacid = 6 ml
molarity of the acid, Macid = ?
The titration formula for acid and base is given as:
Mbase Vbase = Macid Vacid
Macid =
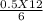
Macid = 1 M
we can see that 1 M solution of HCl was used to neutralize the basic solution of NaOH. The volume of NaOH is 12 ml and volume of HCl used is 6ml.