The temperature of the nitrogen gas is 292.5 K.
Step-by-step explanation:
Given that
Moles of Nitrogen, n = 5 mol
Volume, V = 30 L
Pressure, P = 4 atm
Gas Constant, R = 0.08205 L atm mol⁻¹ K⁻¹
Temperature = ? K
We have to use the ideal gas equation,
PV = nRT
by rearranging the equation, so that the equation becomes,
T =
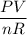
Plugin the above values, we will get,
T =
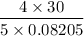
= 292.5 K
So the temperature of the nitrogen gas is 292.5 K.