Answer:
C
Step-by-step explanation:
We know that the pH of A is 1, which means that -㏒[H+] = 1. Solving for [H+], we see that it is equivalent to
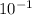 M.
M.
Since the concentrations of [OH-] and [H+] have a product of
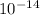 , we can solve for the concentration of OH-:
, we can solve for the concentration of OH-:
[OH-] =
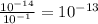
So the concentration of OH- in A is
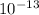 .
.
Now, we see that the pOH of B is 7, which means that -㏒[OH-] = 7. Solving for [OH-], we see that it is equivalent to
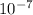 M.
M.
Finally, we can find the difference in concentration between point A and point B by dividing B by A:
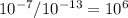
Thus, the answer is C.
Hope this helps!