The molarity of the stock solution is 1.25 M.
Step-by-step explanation:
We have to find the molarity of the stock solution using the law of volumetric analysis as,
V1M1 = V2M2
V1 = 150 ml
M1 = 0.5 M
V2 = 60 ml
M2 = ?
The above equation can be rearranged to get M2 as,
M2 =
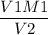
Plugin the values as,
M2 =
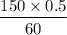
= 1.25 M
So the molarity of the stock solution is 1.25 M.