Mass of Nitrogen is 0.378 g.
Step-by-step explanation:
We have to find the number of moles of nitrogen by using the ideal gas equation, then from the moles we have to find the mass in grams using its molar mass.
PV = nRT
We have to use,
Pressure = 640 mm Hg = 0.84 atm
Volume = 1 L
Temperature = 100° C + 273 = 373 K
R = gas constant = 0.08205 L atm mol⁻¹K⁻¹
n =
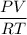
=
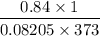
= 0.027 moles
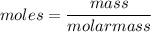
Mass = moles × molar mass
= 0.027 ×14 g/mol = 0.378 g
So the mass of nitrogen is 0.378 g.