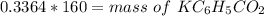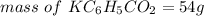Complete Question
A chemistry graduate student is given 125.mL of a 1.00M benzoic acid HC6H5CO2 solution. Benzoic acid is a weak acid with
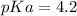 . What mass of KC6H5CO2 should the student dissolve in the HC6H5CO2 solution to turn it into a buffer with pH =4.63? You may assume that the volume of the solution doesn't change when the KC6H5CO2 is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.
. What mass of KC6H5CO2 should the student dissolve in the HC6H5CO2 solution to turn it into a buffer with pH =4.63? You may assume that the volume of the solution doesn't change when the KC6H5CO2 is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits.
Answer:
The mass would be
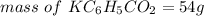
Step-by-step explanation:
From the question we are told that
concentration of benzoic acid is
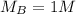
The volume of benzoic acid is
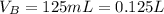
The value of the experimental parameter is
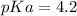
The value of PH is
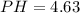
Generally the number of moles is mathematically represented as
No of moles = concentration * volume
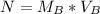
Substituting values
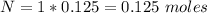
Generally PH is mathematically represented as
![PH = pKa + log([KC_6H_5CO_2])/([HC_6H_5CO_2])](https://img.qammunity.org/2021/formulas/chemistry/college/uabq4mmxbqwsyz603eg3i1rmpdpi9lkqt7.png)
Where
![[KC_6H_5CO_2]](https://img.qammunity.org/2021/formulas/chemistry/college/ffs1i2pvn63fpc4pko54fux2650flj8g4x.png) is the No of moles of
is the No of moles of
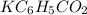 and
and
![[HC_6H_5CO_2]](https://img.qammunity.org/2021/formulas/chemistry/college/8uq332kxqojd0yo1suqem0vgl3g2xiodwl.png) is the number of moles of benzoic acid
is the number of moles of benzoic acid
![4.3 = 4.2 + log([KC_6H_5CO_2])/(0.125)](https://img.qammunity.org/2021/formulas/chemistry/college/bihzv99yrjodavhcq3aomaaz59bb86rakl.png)
![4.63 - 4.2 = log ([KC_6H_5CO_2])/(0.125)](https://img.qammunity.org/2021/formulas/chemistry/college/6u5h1t3rsm2lxzs817cdsnwlmeb2cfi9zp.png)
![0.43 =log ([KC_6H_5CO_2])/(0.125)](https://img.qammunity.org/2021/formulas/chemistry/college/5ug2mv0pdh886z224ntfz1nrcstr7c7kkc.png)
![10^(0.43) =([KC_6H_5CO_2])/(0.125)](https://img.qammunity.org/2021/formulas/chemistry/college/147cl4303j5m2lls7e3x7tzhy7k2bitvh5.png)
![2.6915 *0.125 = [KC_6H_5CO_2]](https://img.qammunity.org/2021/formulas/chemistry/college/x69q6xwrzi62eawcgeri6k0vqehf24j1nv.png)
![[KC_6H_5CO_2]= 0.3364](https://img.qammunity.org/2021/formulas/chemistry/college/fzxrqw22rlti3qu4w8yrt6sm3a6i6bjpep.png)
Generally the number of moles is mathematically represented as
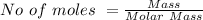
For
![[KC_6H_5CO_2]](https://img.qammunity.org/2021/formulas/chemistry/college/ffs1i2pvn63fpc4pko54fux2650flj8g4x.png)
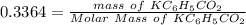
Molar mass of
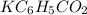 = 160g/mole
= 160g/mole