Answer:
Zero
Explanation:
There are three types of particles inside atoms:
- Proton: the proton is in the nucleus of the atom. It has a positive electric charge, equal to
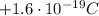 (known as fundamental charge). Its mass is
(known as fundamental charge). Its mass is
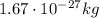 .
.
- Neutron: the neutron is also in the nucleus of the atom, and it has no electric charge. Its mass is slightly larger than the proton's mass, and neutrons and protons are held together inside the nucleus by the strong nuclear force .
Electron: the electron orbits around the nucleus. It has negative electric charge, opposite to the proton (
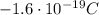 . Its mass is about 1800 times lower than that of the proton.
. Its mass is about 1800 times lower than that of the proton.
Since the neutron has no electric charge the charge of 1 neutron is zero; as a consequence, the charge of 9 neutrons is also zero, as they are all electrically neutral.