Answer:
27.98moles
Step-by-step explanation:
Given parameters:
Number of moles of KClO₃ = 18.65moles
Unknown:
Mass of O₂ = ?
Solution:
The reaction is a decomposition reaction represented with balanced equations as shown below;
2KClO₃ → 2KCl + 3O₂
We have to solve from the known to the unknown. The known here is number of moles of KClO₃ .
From the balanced reaction equation; we have;
2 moles of KClO₃ giving 3 moles of O₂
18.65 moles of KClO₃ will produce
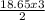 = 27.98moles
= 27.98moles