Answer:
-206.9kJ
Step-by-step explanation:
Heat of combustion = -890kJ/mol
Mass of methane burnt = 3.72g
Unknown:
Heat liberated = ?
Solution:
Heat of combustion is the heat liberated when one mole of an element or compound is burnt completely in excess oxygen gas.
To solve this problem;
Find the number of moles of methane;
Number of moles =

Molar mass of CH₄ = 12 + 4(1) = 16g/mol
Number of moles =
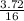 = 0.23moles
= 0.23moles
Now, heat liberated by 0.23mol of methane = -890kJ/mol x 0.23mol
= -206.9kJ