Answer: The molar mass of each gas
Step-by-step explanation:
Mole fraction is the ratio of moles of that component to the total moles of solution. Moles of solute is the ratio of given mass to the molar mass.
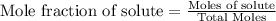
Suppose if there are three gases A, B and C.
a)
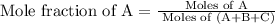
b)
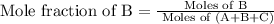
c)
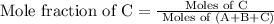
moles of solute =
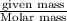
Thus if mass of each gas is known , we must know the molar mass of each gas to know the moles of each gas.