The number of moles of Al₂(SO₄)₃ is 1.2 mol
Step-by-step explanation:
Given:
Volume of the solution, V = 501 mL
V = 0.501L
Molarity of the solution, M = 2.37M
Moles, n = ?
We know:
Molarity =
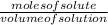
On substituting the values we get:
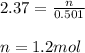
Therefore, the number of moles of Al₂(SO₄)₃ is 1.2 mol