Answer: 10.17 L volume is required for the gases to expand against a constant pressure of 670.
Step-by-step explanation:
The given data is as follows.
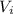 = 0.09 L, P = 670 torr
= 0.09 L, P = 670 torr
As, W = 900 J (given)
so, =
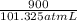
= 8.882 atm L
Now, we will change pressure into atm as follows.
P = 670 torr
=
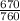 atm
atm
= 0.8816 atm
Therefore, we will calculate the final volume as follows.
W =
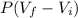
8.882 =
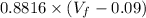
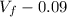 = 10.08
= 10.08
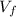 = 10.17 L
= 10.17 L
Thus, we can conclude that 10.17 L volume is required for the gases to expand against a constant pressure of 670.