Answer:
The Ka for propanoic acid (HC3H5O2) is 2.1 x 10⁻⁵
Step-by-step explanation:
Concentration of propanoic acid C = moles / volume in Litres = 0.1 mol / 1 L = 0.1 M
C = 0.1 M
pH = - log [H+]
For weak acids,
[H+] =
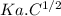
pH = - log (
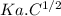 )
)
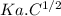 =
=
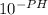
Ka.C = (
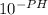 )²
)²
Ka = (
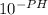 )² / C
)² / C
= (
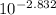 )² / 0.1
)² / 0.1
= 2.1 x 10⁻⁵
Therefore,
Ka of propanoic acid = 2.1 x 10⁻⁵