The boiling point decreases as the volume decreases.
Step-by-step explanation:
The Temperature - Volume law otherwise called as Charles law is applied, which says that the volume of the given gas at constant pressure is directly proportional to the temperature measured in Kelvin. As the volume increases, the temperature also increases, if the volume decreases, then the temperature also decreases.
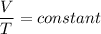
As per the Charles law, here the volume is decreased from 50 ml to 25 ml so the boiling point also decreases.