Answer:
124.91mL
Step-by-step explanation:
Given parameters:
P₁ = 1.08atm
V₁ = 250mL
T₁ = 24°C
P₂ = 2.25atm
T₂ = 37.2°C
V₂ = ?
Solution:
To solve this problem, we are going to apply the combined gas law;
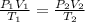
P, V and T represents pressure, volume and temperature
1 and 2 delineates initial and final states
Convert the temperature to kelvin;
T₁ = 24°C, T₁ = 24 + 273 = 297K
T₂ = 37.2°C , T₂ = 37.2 + 273 = 310.2K
Input the variables and solve for V₂
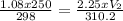
V₂ = 124.91mL