86 percent is the percent yield for this experiment if he expected to produce 5g of product.
Step-by-step explanation:
Given that:
mass of test tube = 5 grams
mass of test tube + reactant is 12.5 grams
mass of reactant = ( mass of test tube + reactant ) - (mass of test tube)
mass of reactant = 12.5 -5
= 7.5 grams
when 7.5 grams of reactant is heated mass of test tube was found to be 9.3 grams.
so mass of product formed = 9.3 - 5
= 4. 3 grams of product is formed (actual yield)
However, he expected the product to be 5 grams (theoretical yield)
Percent yield =
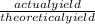 x 100
x 100
putting the values in the formula:
percent yield =
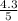 x 100
x 100
= 86 %
86 percent is the percent yield.