The molarity of the solution is 0.14 M/L.
Step-by-step explanation:
Molarity is defined as the quantitative measure of number of molecules of the solute dissolved in per litre of the solution. So it can be calculated by determining the ratio of number of molecules of the solute to the volume of the solution.
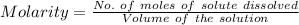
As here it is stated that 0.0171 moles of NaCl is dissolved in 0.122 L of solution, this means the no.of moles of solute is given as 0.0171 and the volume of the solution is 0.122 L.
Then,
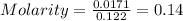
Thus, the molarity of the solution is 0.14 M/L.