Answer:
The maximum amount of potassium carbonate that formed 49.68 g
Step-by-step explanation:
According to question
2 KOH(aq) + CO₂(g) → K₂CO₃(aq) + H₂O(l)
16.2 grams of carbon dioxide are allowed to react with 45.0 grams of potassium hydroxide.
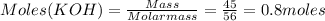
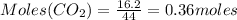
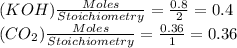
So, CO₂ is limiting reagent.
1 mole CO₂ produce 1 mole K₂CO₃
∴ 0.36 mole CO₂ produce 0.36 mole K₂CO₃ or (0.36 x 138)g = 49.68 g