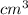Answer:
The volume occupied by the 1000 gm of this mixture is V = 886.82
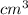
Step-by-step explanation:
Given data
Denote
Chloroform = C and Acetone = A
No. of moles of Acetone =
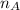
No. of moles of Chloroform =
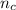
MW of acetone = 58.08 gm
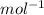
MW of chloroform = 119.37 gm
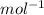
Volume of chloroform = 80.24 ml
Volume of acetone = 74.17 ml
The total number of moles present can be calculated as
1000 g =
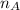 × 58.08 +
× 58.08 +
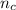 × 119.37
× 119.37
1000 g = n (1-
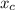 ) × 58.08 + n
) × 58.08 + n
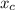 × 119.37
× 119.37
Since
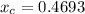
1000 = n (1-0.4693) × 58.08 + n × 0.4693 × 119.37
1000 = n × 0.5307 × 58.08 + n × 56.02
n = 11.515 moles
Therefore
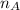 = (1-0.4693) 11.515 = 6.111 moles
= (1-0.4693) 11.515 = 6.111 moles
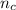 = 0.4693 × 11.515 = 5.404 moles
= 0.4693 × 11.515 = 5.404 moles
Thus the total volume of the solution is given by
V =
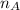
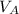 +
+
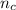
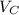
Put all the values in above formula we get
V = 6.111 × 74.166 + 5.404 × 80.235
V = 886.82
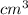
Therefore the volume occupied by the 1000 gm of this mixture is V = 886.82