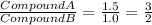Answer:
The mass ratio of oxygen rounded to the nearest whole no. 3 : 2
Step-by-step explanation:
According to Law of Multiple proportion when two elements combine to make two or more different compounds, the mass ratio of the two element in the first compound, when divided by the mass ratio of the second compound , form a simple whole number ratio.
Compound A contains 1.34 g of sulfur for every 0.86 g of oxygen
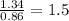
Compound B contains 11.63 g of sulfur for every 10.49 g of oxygen
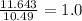
Ratio of oxygen in each compound
always put the larger number over the smaller number.