Answer:
The concentration of
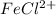 will decrease.
will decrease.
Step-by-step explanation:
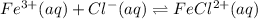
According Le Chatelier’s principle:
- If concentration of products are increased the equilibrium will shift in left direction and vice-versa.
- If concentration of reactants are increased the equilibrium will shift in right direction and vice-versa.
On ad adding silver nitrate to the equilibrium mixture, the silver ion will get combine with chloride ion to precipitate silver chloride which will in return will decrease the concentration of chloride ions.
And due to decrease in concentration of chloride ions the the equilibrium will shift in left direction that is in backward direction to re-establish it self with which concentration of
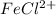 will decrease.
will decrease.