Answer:
pH of the final solution = 3.8
Step-by-step explanation:
Concentration of NaF =
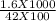 molar
molar
= 0.3 molar
NaF → Na⁺ + F⁻
HF ⇆ H⁺ + F⁻
- NaF is strong electrolyte so completely ionized but HF weak acid not completely ionized.
- Since F⁻ is common ion here
according to common ion effect dissociation of weak acid decreases.
Ka =
![([H]^(+)[F]^(-) )/([HF])](https://img.qammunity.org/2021/formulas/chemistry/college/4prhjess8de80rqaqjoxj9scoaiexll2cl.png)
⇒ [H⁺] =
![(K_(a) [HF])/([F]^(-) )](https://img.qammunity.org/2021/formulas/chemistry/college/3fr5vwg4z6ehhzxwllwmfd5xejcf41n9ho.png) ...............(1)
...............(1)
{Ka of HF = 3.5 x 10⁻⁴} & Concentration of HF = 30 x 4 x 10⁻³ = 0.12 molar
from equation 1
⇒ [H⁺] =
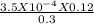 [Concentration of F⁻ ≡ Concentration of NaF]
[Concentration of F⁻ ≡ Concentration of NaF]
⇒ [H⁺] = 0.00014
⇒pH = - log 0.00014 = 3.85