The question is incomplete, complete question is;
A solution of
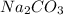 is added dropwise to a solution that contains
is added dropwise to a solution that contains
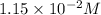 of
of
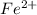 and
and
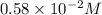 and
and
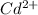 .
.
What concentration of
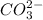 is need to initiate precipitation? Neglect any volume changes during the addition.
is need to initiate precipitation? Neglect any volume changes during the addition.
 value
value
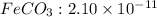
 value
value
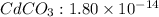
What concentration of
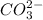 is need to initiate precipitation of the first ion.
is need to initiate precipitation of the first ion.
Answer:
Cadmium carbonate will precipitate out first.
Concentration of
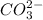 is need to initiate precipitation of the cadmium (II) ion is
is need to initiate precipitation of the cadmium (II) ion is
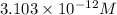 .
.
Step-by-step explanation:
1)
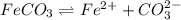
The expression of an solubility product of iron(II) carbonate :
![K_(sp)=[Fe^(2+)][CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/nusdtlt1k0l1e67xrnk835fxug19wehonl.png)
![2.10* 10^(-11)=0.58* 10^(-2) M* [CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/y2iddw6ssgk1k7hbs6lgwkrv2bp13yc2xa.png)
![[CO_3^(2-)]=(2.10* 10^(-11))/(1.15* 10^(-2) M)](https://img.qammunity.org/2021/formulas/chemistry/college/haqdtq9vwrzw8aqm6hnnalhsvgxeuni55v.png)
![[CO_3^(2-)]=1.826* 10^(-9)M](https://img.qammunity.org/2021/formulas/chemistry/college/s1zg6lj1rpq0zol0aacw7stlvw2qo097gw.png)
2)
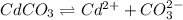
The expression of an solubility product of cadmium(II) carbonate :
![K_(sp)=[Cd^(2+)][CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/xcesv6yxqgx6euu84bnx6hvv9ms0b4q6l5.png)
![1.80* 10^(-14)=0.58* 10^(-2) M* [CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/yxuwz39v5al9df80fa246nbri5mregcnlq.png)
![[CO_3^(2-)]=(1.80* 10^(-14))/(0.58* 10^(-2) M)](https://img.qammunity.org/2021/formulas/chemistry/college/jzi31an574mqhmqp6xbi819vsk4eoah80v.png)
![[CO_3^(2-)]=3.103* 10^(-12) M](https://img.qammunity.org/2021/formulas/chemistry/college/2rgbyoatt2yl2e9m5uljeh4twbqmveqy1q.png)
On comparing the concentrations of carbonate ions for both metallic ions, we can see that concentration to precipitate out the cadmium (II) carbonate from the solution is less than concentration to precipitate out the iron (II) carbonate from the solution.
So, cadmium carbonate will precipitate out first.
And the concentration of carbonate ions to start the precipitation of cadmium carbonate we will need concentration of carbonate ions greater than the
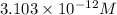 concentration.
concentration.