8 moles of CaO produced from 4 moles O2.
Step-by-step explanation:
Balanced chemical equation for the reaction:
2 Ca + O2 --> 2 CaO
Data given:
moles of O2 = 4
Moles of CaO =?
From the reaction it is seen that 1 mole of O2 is used in the reaction to produce 2 moles of CaO
hence, 4 moles of O2 will give x moles of CaO
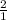 =
=
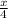
x =8
So, 8 moles of CaO will be produced when 4 moles of O2 will be used in the reaction. Since oxygen is the limiting reagent in the reaction.