Answer:
Work done in this process = 4053 J
Step-by-step explanation:
Mass of the gas = 0.092 kg
Pressure is constant = 1 atm = 101325 pa
Initial temperature
 = 200 K
= 200 K
Final temperature
 = 200 - 85 = 115 K
= 200 - 85 = 115 K
Gas constant for nitrogen = 297
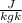
When pressure of a gas is constant, volume of the gas is directly proportional to its temperature.
⇒ V ∝ T
⇒
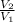 =
=
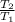 ------------ ( 1 )
------------ ( 1 )
From ideal gas equation

 = m R
= m R
 ------ (2)
------ (2)
⇒ 101325 ×
 = 0.092 × 297 × 200
= 0.092 × 297 × 200
⇒
 = 0.054
= 0.054

This is the volume at initial condition.
From equation 1
⇒
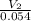 =
=
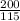
⇒
 = 0.094
= 0.094

This is the volume at final condition.
Thus the work done is given by W = P [
 -
-
 ]
]
⇒ W = 101325 × [ 0.094 - 0.054]
⇒ W = 4053 J
This is the work done in that process.