Answer:
The salt (Sodium Nitrate) is prepared by the reaction of nitric acid and sodium hydroxide. The reaction is also known as neutralization reaction.
Step-by-step explanation:
The reaction between nitric acid (
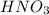 ) and sodium hydroxide (NaOH) will result in the formation of salt (Sodium nitrate). The balanced reaction between nitric acid and sodium hydroxide is shown below
) and sodium hydroxide (NaOH) will result in the formation of salt (Sodium nitrate). The balanced reaction between nitric acid and sodium hydroxide is shown below
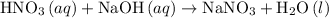
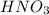 is a strong acid and NaOH is a strong base. This reaction is an example of neutralization reaction.
is a strong acid and NaOH is a strong base. This reaction is an example of neutralization reaction.