Answer:
The value of maximum pressure the balloon could be filled = 6.40 atmosphere.
Step-by-step explanation:
When helium from the cylinder is filled inside the balloon the heat & work
transfer is zero during the process. so from the first law of thermodynamics
change in internal energy is zero.
⇒
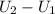 = 0
= 0
⇒
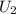 =
=
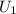
⇒
 =
=
 ------------- (1)
------------- (1)
Final temperature is equal to initial temperature.
Equation (1) can be written as
⇒
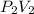 =
=
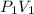 -------------(2)
-------------(2)
Initial volume = 44 L
Final volume = 296 L
Initial pressure = 43.1 atmosphere
Final pressure = we have to calculate
Put all the values in Equation (2) we get,
⇒
 × 296 = 43.1 × 44
× 296 = 43.1 × 44
⇒
 = 6.40 atmosphere
= 6.40 atmosphere
This is the value of maximum pressure the balloon could be filled.