Answer:
The molality of copper(II) acetate in the solution is 0.3960 mol/kg.
Step-by-step explanation:
Mass of copper(II) acetate = 26.4 g
Molar mass of copper(II) acetate = 181.63 g/mol
Moles of copper(II) acetate =-
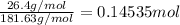
Mass of water = 367 g = 367 × 0.001 kg ( 1 g = 0.001 kg)
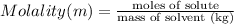
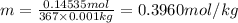
The molality of copper(II) acetate in the solution is 0.3960 mol/kg.