Answer:
5.53 grams is the mass of NaOH in the mixture.
Step-by-step explanation:
Let the mass of NaOH be x and
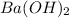 be y.
be y.
x + y = 10.1 g..[1]
Moles of NaOH =
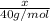
Moles of
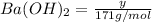
Moles of HCl = n
Volume of HCl solution = 107.8 mL = 0.1078 L( 1 mL = 0.001 L)
Molarity of HCl solution = 1.53 M
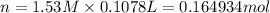
0.38709 moles neutralizes all the NaOH and
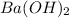 present in solution.So, This manes that;
present in solution.So, This manes that;
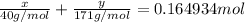
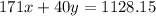 ..[2]
..[2]
On solving [1] and [2] we get ;
x = 5.53 g
y = 4.57 g
5.53 grams is the mass of NaOH in the mixture.