Answer:
9.04 g
Step-by-step explanation:
Given that:
pH of NaOH = 13.40
pOH = 14 - pH
pOH = 14 - 13.40
pOH = 0.6
Now, from there we can find the concentration of NaOH = [OH⁻]
=
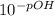
=
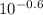
= 0.2512 M
Given that; volume = 0.9 L
∴ number of moles of NaOH = volume × concentration of NaOH
= 0.9 × 0.2512
= 0.2261 moles
mass of NaOH = number of moles of NaOH × molar mass of NaOH
mass of NaOH = 0.2261 × 40
mass of NaOH = 9.04 g