Answer: The EMF of the cell is -0.100 V and the reaction is non-spontaneous
Step-by-step explanation:
The half reaction for the equation follows:
Oxidation half reaction:
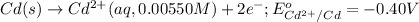
Reduction half reaction:
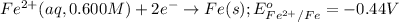
Net cell reaction:
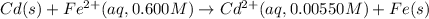
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
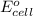 of the reaction, we use the equation:
of the reaction, we use the equation:
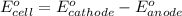
Putting values in above equation, we get:
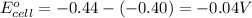
- To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Cd^(2+)])/([Fe^(2+)])](https://img.qammunity.org/2021/formulas/chemistry/college/hrka4mb9mkzhu83o56pg4b7biaiaics2p0.png)
where,
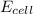 = electrode potential of the cell = ?
= electrode potential of the cell = ?
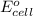 = standard electrode potential of the cell = -0.04 V
= standard electrode potential of the cell = -0.04 V
n = number of electrons exchanged = 2
![[Cd^(2+)]=0.00550M](https://img.qammunity.org/2021/formulas/chemistry/college/v5dzadvj8nvudza2o4usnsw2ic7cz6ogzn.png)
![[Fe^(2+)]=0.600M](https://img.qammunity.org/2021/formulas/chemistry/college/18jqu4mvkn1ngq1chjq1wkj9bzh43c2dqc.png)
Putting values in above equation, we get:
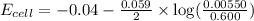
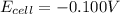
For the reaction to be spontaneous, the Gibbs free energy of the reaction must come out to be negative.
Relationship between standard Gibbs free energy and standard electrode potential follows:
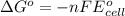
For a reaction to be spontaneous, the standard electrode potential must be positive.
Hence, the EMF of the cell is -0.100 V and the reaction is non-spontaneous