Answer:
A pressure value more than 0.012 atm must be applied to the apparatus at 20.0°C to produce pure water.
Step-by-step explanation:
When pressure more than the value of osmotic pressure of the solution is applied to the solution then water will move out from the solution which is termed as reverse osmosis.
Given that 1.70 grams of sucrose was present in 1 L of solution.
Moles of sucrose present in 1 liter of solution =
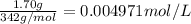
C = 0.004971 mol/L
Temperature of the solution = T = 20.0°C = 20.0+273 K = 293 K
Osmotic pressure of soluion =

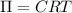
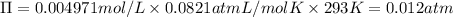
A pressure value more than 0.012 atm must be applied to the apparatus at 20.0°C to produce pure water.