The volume will be 674 ml.
Step-by-step explanation:
As the temperature is said to be constant, then the present system will be obeying Boyle's law. So according to this law, the volume of a gaseous system is inversely proportional to the pressure of the system at constant temperature and number of moles.
So according to this law,
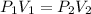
As here the initial pressure is 3.52 atm and the initial volume is 354 mL with the reduced pressure as 1.85 atm. Since, there is a decrease in the pressure, there tends to increase in the volume.
So,
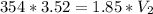
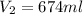
So, the volume will be 674 ml.