Answer:
Machine used 392 MJ energy as mechanical energy.
Step-by-step explanation:
Efficiency(e) is defined as the ratio of the work done by the machine to the energy provided to the machine.
 .....(1)
.....(1)
The efficiency of the machine is also given by the relation :
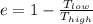 .....(2)
.....(2)
Here T(low) and T(high) denotes the temperature in kelvin.
According to the problem, T(low) = 35⁰ C = 35 + 273 = 308 K
T(high) = 330⁰ C = 330 +273 = 603 K
Substitute the values of T(low) and T(high) in the equation (2).
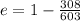

e = 0.49
According to the problem, Energy Given = 800 MJ
Substitute the value of efficiency(e) and Energy Given in the equation (1).
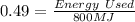
Energy used = 0.49 x 800 MJ = 392 MJ