Answer:
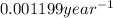 is the rate constant for this reaction.
is the rate constant for this reaction.
It will take
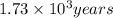 to concentration to reach 12.5% of its original value.
to concentration to reach 12.5% of its original value.
Step-by-step explanation:
A decomposition reaction follows first order kinetics:
Half life of the reaction =
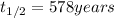
Rate constant of the reaction = k
For first order reaction, half life and rate constant are linked with an expression :
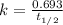
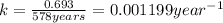
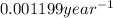 is the rate constant for this reaction.
is the rate constant for this reaction.
Initial concentration of reactant =
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = x
= x
Final concentration of reactant after time t =
![[A]](https://img.qammunity.org/2021/formulas/chemistry/college/3c3qqp51mqk0xw1nmfldczp98fmi09kbrv.png) = 12.5% of x = 0.125x
= 12.5% of x = 0.125x
The integrated law of first order reaction :
![[A]=[A_o]* e^(-kt)](https://img.qammunity.org/2021/formulas/chemistry/college/9w29d3l2dibvmc74mm6nsie5d3ot0cl7py.png)
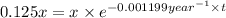
t = 1,734.31 years =
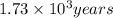
It will take
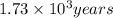 to concentration to reach 12.5% of its original value.
to concentration to reach 12.5% of its original value.