Answer:
35.1 kJ/mol is the expected value for the heat of sublimation of acetic acid.
Step-by-step explanation:
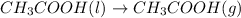 ..[1]
..[1]
Heat of vaporization of acetic acid =
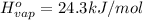
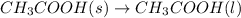 ..[2]
..[2]
Heat of fusion of acetic acid =
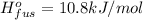
Heat of sublimation of acetic acid =
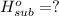
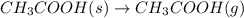 ..[3]
..[3]
[1] + [2] = [3] (Hess's law)
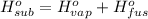
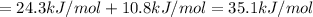
35.1 kJ/mol is the expected value for the heat of sublimation of acetic acid.