Question is incomplete, complete question is:
Assuming that you recovered 100% of the acetanilide in the sample, how much of the mass of the original sample was made up of impurities?
Mass of impure sample = 1.728 g
Mass of purified crystals = 0.201 g
Answer:
1.527 grams of impurity is present in 1.728 grams of impure sample.
Step-by-step explanation:
Mass of sample = 1.728 g
Mass of pure crystal of acetanilide = 0.201 g
Mass of acetanilide we want to collect = 0.201 g
Percentage recovery of the acetanilide = 100%
Mass of impurity = m
100% recover means that all the acetanilide has been crystallized into pure compound. So, amount of sample will be equal to sum of mass of an impurity and mass of pure crystal.
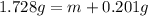
m = 1.728 g - 0.201 g = 1.527 g
1.527 grams of impurity is present in 1.728 grams of impure sample.