Answer:
3.61mole of NaCl
Step-by-step explanation:
Given parameters:
Mass of Na = 83g
Unknown:
Number of moles of NaCl produced = ?
Solution:
We write the reaction equation first;
2Na + Cl₂ → 2NaCl
Since Cl₂ is in excess in this reaction, the extent will be determined by how much Na is available.
find the number of Na and relate to that of NaCl
Number of moles of Na =

molar mass of Na = 23g/mole
=
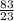
= 3.61mole
2 mole of Na reacted with 2 mole of NaCl
3.61 mole of Na will react with 3.61mole of NaCl