Answer: 2.

Step-by-step explanation:
Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound.
a) If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of N = 30.4 g
Mass of O= 69.6 g
Step 1 : convert given masses into moles
Moles of N=
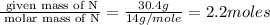
Moles of O=
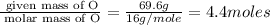
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For N =
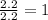
For O =
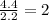
The ratio of N: O = 1; 2
Hence the empirical formula is
