Answer: The molar mass of the unknown compound is 18765.7 g/mol
Step-by-step explanation:

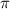 = osmotic pressure = 0.0105 atm
= osmotic pressure = 0.0105 atm
C= concentration in Molarity
R= solution constant = 0.0821 Latm/Kmol
T= temperature = 300 K
For the given solution: 200 mg or 0.2 grams of unknown compound is dissolved in 25 ml of solution.
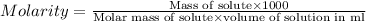
Putting in the values we get:
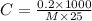
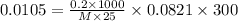
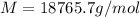
The molar mass of the unknown compound is 18765.7 g/mol