1.2 x
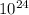 number of atoms in
number of atoms in
 molecule
molecule
Step-by-step explanation:
- As we can see, one molecule of hydrogen is existing as two hydrogen atoms bond together. So, as we know that the number of molecules in one mole of the hydrogen atom is the Avogadro's number
- Here, there are two atoms in every molecule of hydrogen. Hence, the number of atoms in every molecule of hydrogen would be calculated by multiplying 2 with the Avogadro's number which gives the answer given above.
- Hence, 1.2 x
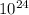 the number of atoms in every hydrogen molecule.
the number of atoms in every hydrogen molecule.