Answer:
Following the already established formula as represented below, the molal concentration of the same solution is 1.034m.
Step-by-step explanation:
Using the formula -
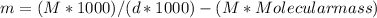
Where,
m = molality = What we are looking for?
d = density = 1.202 g/mL
M = Molarity = 0.926M
Molecular mass for lead(II) nitrate = 331.2 g/mol
Substituting the values in the question into the equation, we have
m = (0.926 * 1000) / (1.202 * 1000) - (0.926 * 331.2)
solving for the numerator
= (0.926 * 1000) = 926
solving for the denominator
= (1.202 * 1000) - (0.926 * 331.2) = 1202 - 306.6912 = 895.3088
solving for m = molality.........
= 926/895.3088
m = 1.034m approximated to three decimal places