Answer : The amount of HF left was, 76.0 grams.
Explanation :
The balanced chemical reaction is:
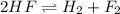
Initial amount 80.0g 0g 0g
At eqm. (80.0-2x)g (x)g (x)g
As we are given that:
Amount of
 at equilibrium = 2.0 g
at equilibrium = 2.0 g
That means,
x = 2.0 g
Now we have to determine the amount of HF that was left if equilibrium was reached when 2.0 g of hydrogen had been produced.
Amount of HF left = (80.0 - 2x) g
Put x = 2.0, we get:
Amount of HF left = (80.0 - 2\times 2.0) g
Amount of HF left = 76.0 g
Thus, the amount of HF left was, 76.0 grams.