Step-by-step explanation:
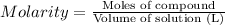
Moles of compound =
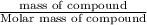
We have ;
Volume of solution = 600 mL = 0.600 L ( 1 mL = 0.001 L)
Moles of NaOH = n
Molarity of the solution = 3 M
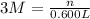
n = 3 M × 0.600 L = 1.800 mol
Mass of 1.800 mole sof NaOH :
1.800 mol × 40 g/mol = 72.0 g
Preparation:
Weight 72.0 grams of sodium hydroxide and add it to the 500 mL of volumetric flask along with some water. Dissolve the all the solute by adding small proportion of water. After the solution becomes clear make the water upto the mark of 500 ml.
Transfer the solution to a bigger beaker and 100 mL of water more to it.