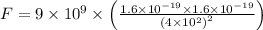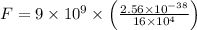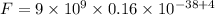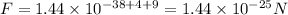The value of electric force between two protons is
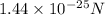
Step-by-step explanation:
The given question looks to be incomplete, so guess the question might be about to find the value of electric force between two protons.
Coulomb's law states that two same charges will get repelled and attracted for opposite charges. The electric force would be directly proportionate to the charges of two and an inversely proportionate to the distance separation between them. The equation can represent as below,
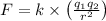
Where,
F – Electric force
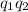 – Charges
– Charges
k – Coulomb constant =
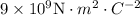
r – Distance between two charges
In given question,
r -
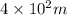
Charge of proton = q =
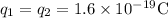
Substitute all the known and given values, we get